Amperometric miniaturized enzyme-based nanobiosensor for sensitive detection of sarcosine -potential prostate cancer marker in urine samples
Amperometric miniaturized enzyme-based nanobiosensor for sensitive detection of sarcosine -potential prostate cancer marker in urine samples
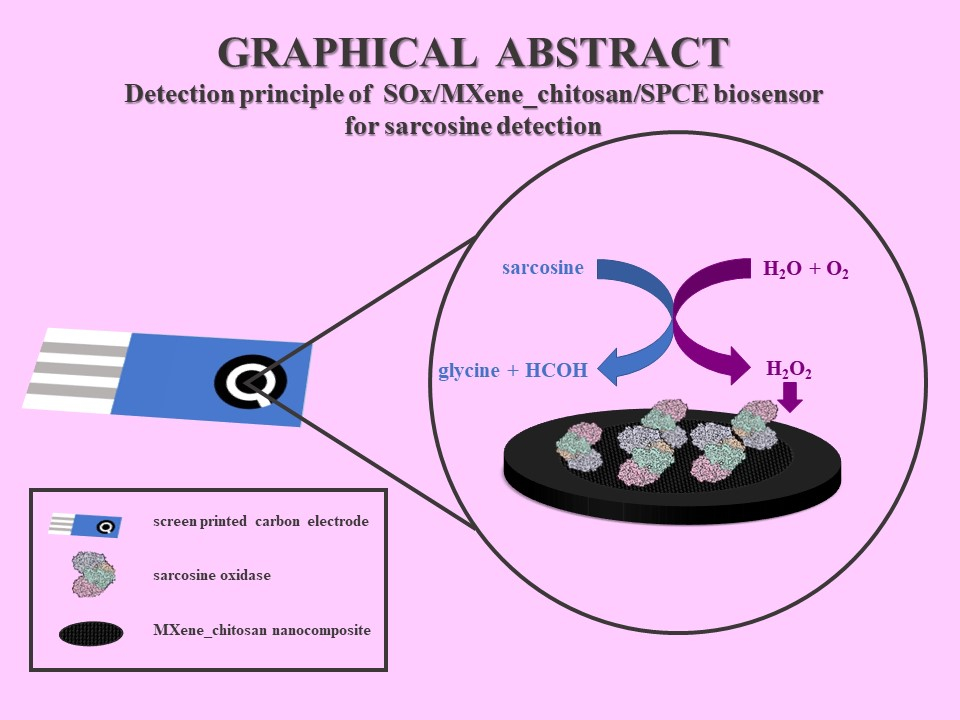
Cancer continues to be a worldwide killer, despite enormous amount of research seen during the past decade. Among men, prostate cancer (PCa) is the most frequently diagnosed cancer disease. Currently, the gold standard in PCa diagnostics is analysis of the prostate specific antigen (PSA) level in blood serum. PSA level is low in healthy men with an increased level found as a result of having either PCa as well as prostate inflammation or benign prostate hyperplasia. Thus, PSA is prostate specific rather than cancer specific. For this purpose, there is a need to find new, more specific biomarkers. This work is focused on the detection of a sarcosine, which can be found in elevated levels in urine during PCa [1]. In this work, 2D nanomaterial MXene Ti3C2Tx was used to immobilise enzyme sarcosine oxidase (SOx) onto the surface of screen-printed carbon electrodes (SPCEs) to fabricate a miniaturized SOx/MXene-chitosan/SPCE nanobiosensor. Quantification of sarcosine was achieved indirectly via amperometric detection of H2O2 produced during the enzymatic reaction. Miniaturized sarcosine nanobiosensor displayed low detection limit of 7.0 nM. Fitting of calibration curve using Hill model and Michaelis-Menten kinetics rendered the following maximum peak current output Imax: (4.10 ± 0.35).10-5 A. The prepared device was further successfully used for sarcosine determination in artificial urine with a recovery index 105.7%.
The authors would like to acknowledge the financial support received from the Slovak Research and Development Agency APVV 20-0476 and APP 17-0300 and from the Slovak Scientific Grant Agency VEGA 2/0137/18 and 2/0130/20. We would like to acknowledge the support received from the Ministry of Health of the Slovak Republic under the project registration number 2019/68-CHÚSAV-1.
[1] Bertók T. et al.; Novel Prostate Cancer Biomarkers: Aetiology, Clinical Performance and Sensing Applications, Chemosensors, 2021, Vol. 9, art. No. 205.
Very impressive work,
Very impressive work, congratulations! Can you, please, theoretically specify the developmental stages within the process of inventing new diagnostic method for prostate cancer? I mean from testing the nanosensor in experimental conditions up-to-the medical applications approved by validation authorities. How long does it take, what are the necessary phases, how much does it cost? Estimate just according to your opinion... Thanks in advance for fruitful discussion, ZB
Dear Dr. Brnoliaková, firstly
Dear Dr. Brnoliaková, firstly I would like to thank you for your comment.
Many issues and aspects need to be addressed during product commercialization. The first issue that has to be solved is intellectual property, usually in the form of a patent, that gives you the legal right to exclude others from making, using, or selling your invention for a limited period of time in exchange for publishing an enabling disclosure of the invention. Protection of IP is a process for several years, especially if the patent is approved in various countries of the world. This is also enormously expensive and important in terms of obtaining venture capital, as investors do not normally like to share IPs with multiple parties (eg interuniversity cooperation). This is followed by testing in the case of drugs, or validation in the case of diagnostics. This step is difficult to obtain/collect volunteers or samples. In the final, it is necessary to obtain several certificates - ISO9001 for quality management and ISO13485 for medical devices, the CE mark if you want to offer it on the market. In parallel, you must also develop cooperation with workplaces (labaks, clinics), where the test would soon be offered. Initially, you do it privately, if you get overpayed by insurance companies and push your diagnostics into the guideline, then you can provide your diagnostics where you want, either as a service (samples go to you, you have to have a strong infrastructure) or sell a kit (for this you must have a production hall and other certificates, GMP-good manufacturing practice; you can also contract another manufacturer and outsource this process). If you want to go out of EU, in addition to EMA (at SVK it is ŠÚKL) you must also obtain FDA approval. And finally - if you finally get your product to the market, it will set the biggest fight. The market is usually dominated by several large companies (eg Roche) and you have to spend enormous funds on marketing and sales to push the product to the market.
Thanks for discussion. If you have any questions, feel free to ask me.
Ing. Štefánia Hrončeková
Dear Dr. Hroncekova, thank
Dear Dr. Hroncekova, thank you so much for your eligible, careful and highly organized reply. I wish you all the best in your future research career. Cordially, ZB